Our climate hysteria “friends” tell us that solar panels and windmills will save the planet. And from the COP28 conference (under their point 28-a), we learn that the powers-that-be want to triple global renewable energy "capacity" by 2030. However, wind turbines and solar photovoltaic panels are intermittent and variable. Their average load factor over five years in the EU is 23% and 11% respectively. A wind turbine therefore needs to be accompanied by a controllable power plant (What? Is that a conventional petrochemical power plant? Just wondering...) for more than three quarters of the time, and a solar farm for 90% of the time.
To solve that sticky problem, many solar energy pushers want to install backup battery banks (using lithium ion batteries for the most part) to enhance "reliability" by increasing the load factor. As an example, the solar panel farm proposed for Rancho Viejo near Santa Fe in New Mexico planned to use 69 large shipping containers full of lithium ion batteries to store the electricity. The Fire Departments in all nearby locations (city and county) declared they would be unable to fight any fires that break out, leaving the nearby residents extremely vulnerable.
In addition, just one day of global backup using Elon Musk's Megapacks would require a $190 Trillion investment, which is twice Global GDP! And how many lithium batteries is that exactly? And what's the environmental cost of mining and manufacturing all that lithium and other materials needed for the batteries? Ok, getting ahead of myself here. Let's get back to lithium fires. But first, what are lithium ion batteries and how do they work?
While most handheld electronics use lithium ion batteries with a polymer gel as the electrolyte, larger lithium ion batteries have liquid electrolytes such as LiPF6, LiBF4, or LiClO4 in an organic solvent. The performance of these batteries is strongly dependent on the electrochemical performance of the electrolytes, so much current research goes into developing those materials, which seem to be getting much more exotic and perhaps hazardous at the same time!
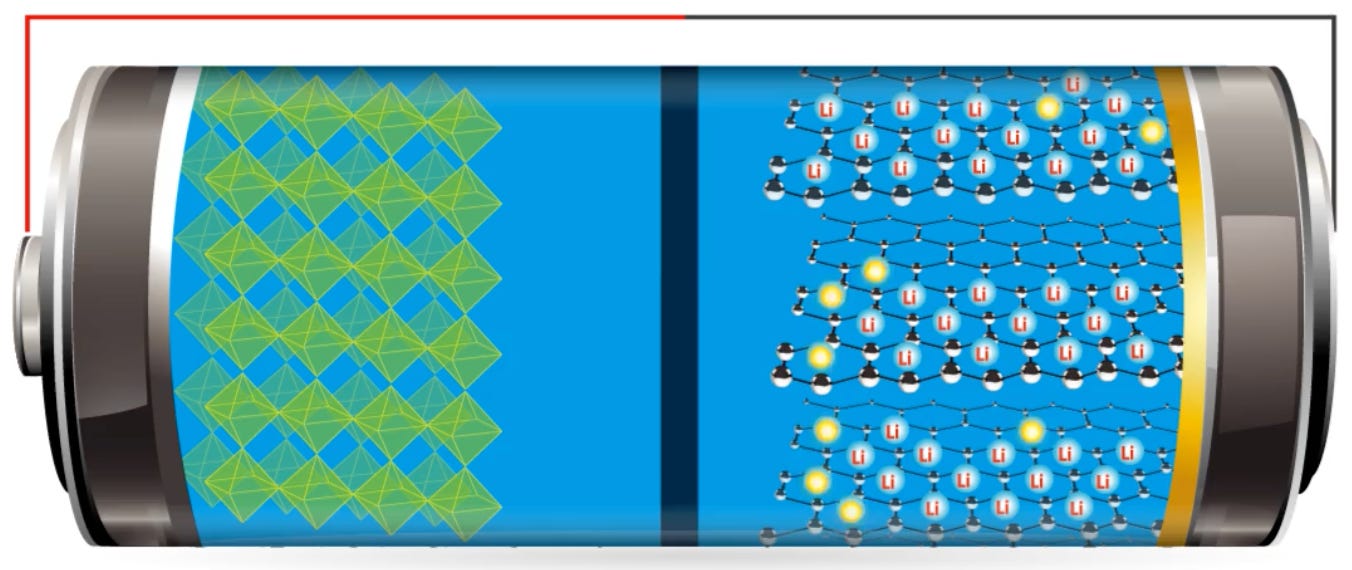
A lithium ion battery (see figure above) is constructed with an anode, cathode, separator, electrolyte, and two current collectors (positive and negative). The anode and cathode store the lithium. The electrolyte carries positively charged lithium ions from the anode to the cathode through the separator during discharge. The movement of the lithium ions creates free electrons in the anode which creates a charge at the positive current collector. The electrical current then flows from the current collector through a device being powered (cell phone, computer, etc.) to the negative current collector. The process basically reverses during charging, moving the lithium ions back to the anode to start the process all over again.
Okay, fine. Let’s get back to the main subject. How does lithium burn?
Lithium is a metal with a very high oxidation potential, so that it can oxidize nearly anything else around it. If one cell within a lithium battery has a problem or is compromised in some way, the heat released can easily lead to "thermal runaway" with neighboring cells catching fire etc., etc., until all the cells are used up, or in the case of this NBC New York test, an explosion happens.
So, why not just use a home fire extinguisher and put the fire out? It turns out that lithium is highly reactive with water and forms strong hydroxide solutions, yielding lithium hydroxide (LiOH) and hydrogen gas (probably not a good thing to have around a fire?).
Fortunately, lithium ion batteries do not contain metallic lithium, but they do contain lots of other combustible materials (electrolytes with their organic solvents and cathode/anode materials, as well as the current collectors) that burn at high temperature and don't need a lot of oxygen. One fire service recommends Class B fire extinguishers.
In my humble opinion, however, with the danger of thermal runaway and explosion, it's probably best to vacate the area entirely and call in the professionals! Tell me in the comments what you think!
References
https://clintel.org/cop28-a-revival-of-nuclear-energy-and-a-bright-future-for-fossil-fuels/
https://www.aes.com/new-mexico/project/rancho-viejo-solar
https://www.energy.gov/energysaver/articles/how-lithium-ion-batteries-work
https://www.nbcnewyork.com/investigations/i-team-anatomy-of-a-lithium-ion-battery-fire/4284013/
Y. Okamoto and Y. Kubo, Ab Initio Calculations of the Redox Potentials of Additives for Lithium-Ion Batteries and Their Prediction through Machine Learning, ACS Omega 2018, 3, 7, 7868–7874 https://pubs.acs.org/doi/10.1021/acsomega.8b00576